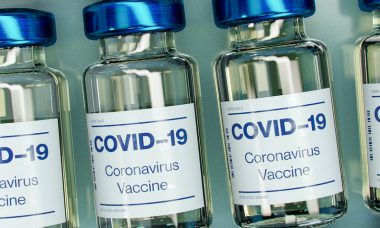
The agency encourages reporting of illegally marketed substances. The U.S. Food and Drug Administration (FDA) has issued a warning for the public and healthcare providers regarding the risks…
Read MoreFood and Drug Administration
Senators Urge HHS to Expand Coverage for OTC Contraceptives
Many birth control pills are covered but require a prescription. Forty-eight senators wrote a letter to President Biden’s administration this week, advocating for healthcare to expand coverage to…
Read MoreFDA Launches Pilot Program to Develop Rare Disease Therapies
A limited number of sponsors will be allowed to participate. The U.S. Food and Drug Administration (FDA) has launched the Support for clinical Trials Advancing Rare disease Therapeutics…
Read MoreFDA Updates Guidance to Guard Against Mislabeling Food Allergens
New guidelines provide strategies to protect consumers and ensure accurate allergen labeling. The U.S. Food and Drug Administration (FDA) has updated draft guidance to assist food facilities in…
Read MoreFDA Modernizes Regulation and Evaluation of Animal and Veterinary Products
The new agenda outlines the objectives of the Center for Veterinary Medicine. The U.S. Food and Drug Administration (FDA) announced modernization of its approach to innovative animal and…
Read MoreCDC Issues Universal Recommendation for Updated COVID-19 Vaccines
FDA recently approved updated shots from Moderna and Pfizer. Centers for Disease Control and Prevention (CDC) Director Mandy Cohen has approved a universal recommendation for Moderna and Pfizer’s…
Read MoreFDA Authorizes Pfizer’s and Moderna’s Updated COVID-19 Vaccines
Officials say the update is formulated to address the most recent subvariant. The Food and Drug Administration (FDA) has authorized Pfizer and Moderna’s updated COVID-19 vaccines, to address…
Read MoreHHS Recommends Easing Marijuana Restrictions
Recommendation prompts the DEA to conduct a final review for reschedulization. The Department of Health and Human Services (HHS) has announced that the department recommends the reclassification of…
Read MoreFDA Issues Warning Letters to Three Infant Formula Manufacturers
The agency is urging companies to correct manufacturing processes to prevent contamination. The Food and Drug Administration (FDA) sent warning letters to three infant formula companies, telling them…
Read MoreUSDA Investment of $12.5M Fuels Tech Innovation in Agriculture
The National Institute of Food and Agriculture director says small businesses play a key role in food and agriculture innovation. The U.S. Department of Agriculture (USDA) announced an…
Read More