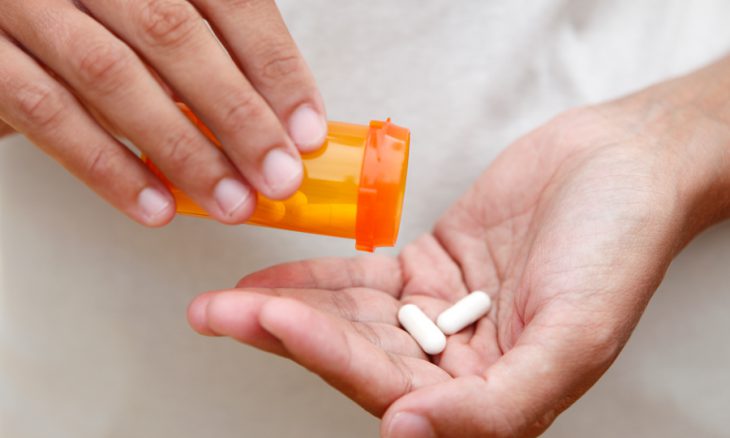
A lawsuit challenges the FDA approval process in 2000.
Attorneys general from 44 states recently met to discuss a lawsuit filed against the Food and Drug Administration (FDA) in 2021 seeking to block nationwide access to the abortion pill mifepristone. Pro-life groups filed the lawsuit to overturn the FDA’s approval of the drug in 2000, claiming that the process they used to approve the drug was “improper” and did not adequately consider its safety. The groups filed their lawsuit in the federal court of Amarillo, Texas, to be heard by U.S. District Judge Matthew Kacsmaryk.
Attorneys general advocating the right to life agreed that the FDA improperly approved mifepristone for public use. The attorneys general in favor of abortion said that even if there was not “overwhelming medical consensus” confirming the drug’s safety, the lawsuit was filed far too late to overturn an official decision made in 2000. Legal scholars, congressional leaders, and policy advocates also submitted briefs to the Amarillo federal court weighing in on the lawsuit.
As the Lord Leads, Pray with Us…
- For wisdom for Judge Kacsmaryk as he hears the case and considers the arguments.
- For the state attorneys general as they pursue their restrictions or support regarding abortion procedures, including pills.
- For health officials to be transparent about the effects of mifepristone.
Sources: Reuters, NBC News